Because the erythrocyte-bound zonisamide is inactive and binding varies with blood concentration the. It is also soluble in methanol ethanol ethyl acetate and acetic acid.

Zonisamide Usp At Rs 17500 Kg Active Pharmaceutical Ingredients Id 21457372948
Zonisamide may act by blocking repetitive firing of voltage-gated sodium channels leading to a.
Active form of zonisamide. The active substance is zonisamide an established active substance described in the United States Pharmacopoeia USP. Approximately 88 of circulating zonisamide is bound in erythrocytes. Zonisamide may be a carbonic anhydrase inhibitor although this is not one of the primary mechanisms of action.
Zonisamide DESCRIPTION ZONEGRAN zonisamide is an antiseizure drug chemically classified as a sulfonamide and unrelated to other antiseizure agents. The metabolites which could not be detected in plasma are devoid of anticonvulsant activity. 100 mg Applicant Accord Healthcare BV.
Parent drug and SMAP can additionally be glucuronidated. The active ingredient is zonisamide. The active ingredient gives the desired therapeutic effect whereas the inactive ingredient helps in making the medicine stable.
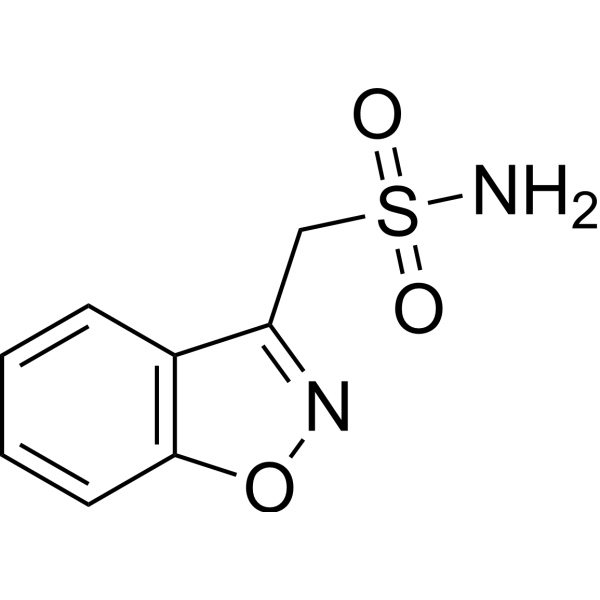
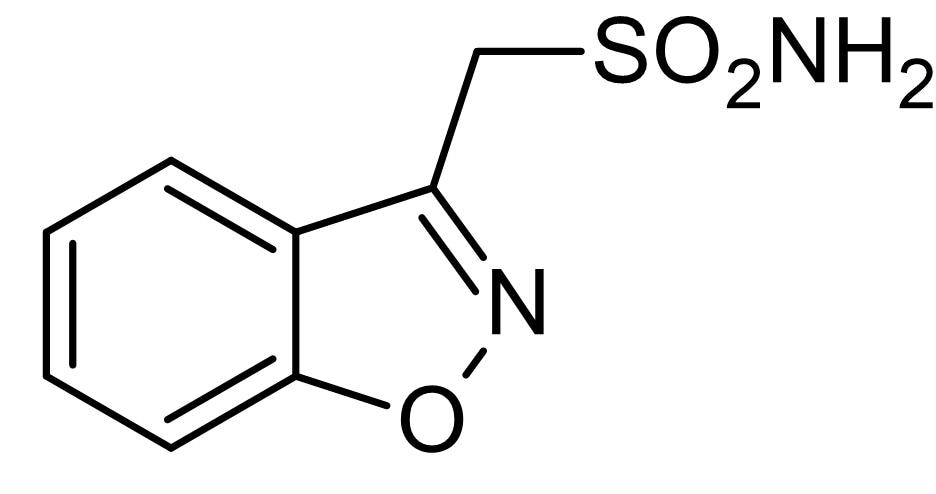
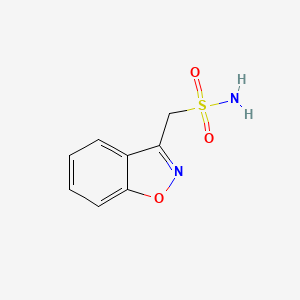
Zonisamide Zonegran C 8 H 8 N 2 O 3 S MW 21223 occurs as a white to off-white powder that melts at about 163C. It is tasteless and odorless has a pKa of 102 and is moderately soluble in water 080 mgmL and 01 N HCl 050 mgmL. Zonisamide is a white powder pKa 102 and is.
VALPROATE Depakene Fully ionized at body pH thus active form is valproate ion. To develop simple accurate precise and cost effective UV-VIS Spectrophotometric method for the estimation of Zonisamide in bulk and pharmaceutical dosage form. The medicines below all contain the following active ingredients.
Zonisamide Zonisamide is a sulfonamide anticonvulsant approved for use as an adjunctive therapy in adults with partial-onset seizures. The exact mechanism of action is not known but zonisamide has been shown to block voltage sensitive sodium and calcium channels which is thought to. The active substance is zonisamide a benzisoxazole derivative.
Zonisamide is an antiseizure drug chemically classified as a sulfonamide and unrelated to other antiseizure agents. Metabolites are not active. Beginning of content Active ingredient.
EU-Procedure number original DEH4382001-003. Zonisamide 20 mg per 1 ml. Active Ingredients ZONISAMIDE 100 mg1.
Active Ingredient Drug Product Dosage Form Reasons Comments Valproic acid Depakote Depakote ER bTabletCapsule Teratogen j Venlafaxine XR h Effexor XR Capsule Extended-release b Verapamil SR Calan SR Tablet Slow-release bf Vitamin A A-25 Capsule Liquid filled d Ziprasidone Geodon Capsule Genotoxin carcinogen teratogen j Zonisamide Zonegran. The active ingredient is zonisamide 12-benzisoxazole 3-methanesulfonamide. Doses are various strengths of the medicine like 10mg 20mg 30mg and so on.
Its amides and esters are also active. Active ingredients Size Unit NHS indicative price Drug tariff Drug tariff price. Zonisamide may act by blocking repetitive firing of voltage-gated sodium channels leading to a reduction of T-type calcium channel currents or by binding allosterically to GABA receptors.
Zonisamide binds to erythrocytes. Zonisamide is an antiepileptic compound chemically unrelated to other antiepileptic drugs AEDs. You can select a medicine from this list to find out more - including side effects age restrictions food interactions and whether the medicine is subsidised by the government on the pharmaceutical benefits scheme PBS.
One of a series of carboxylic acids with antiepileptic activity. Ring of the parent drug by CYP3A4 to form 2-sulphamoylacetylphenol SMAP and also by N-acetylation to form N-acetyl zonisamide. The metabolites which could not be detected in plasma are devoid of anticonvulsant activity.
Zonisamide is a white to off-white powder which is highly soluble in water freely soluble in dimethylformamide and sparingly. 23 D-40764 Langenfeld Germany RMS Germany Date of last change 09112021 ATC-Code N03AX15 Zonisamide. Active Substances zonisamide 100 mg.
Zonisamide 20 mg per 1 ml. Both active ingredients and inactive ingredients form the composition. The present invention provides an improved process for the preparation of zonisamide or a derivative thereof comprising a reacting 12-benzisoxazole-3.
Zonisamide is the pharmacologically active agent. There is no evidence that zonisamide induces its own metabolism. Zonisamide is metabolised primarily through reductive cleavage of the benzisoxazole ring of the parent drug by CYP3A4 to form 2-sulfamoylacetylphenol SMAP and also by N acetylation.
Zonisamide may be a carbonic anhydrase inhibitor although this is not one of the primary mechanisms of action. The active ingredient is zonisamide 12-benzisoxazole-3-methanesulfonamide. Active ingredients Size Unit NHS indicative price Drug tariff Drug tariff price.
ZONEGRAN zonisamide is an antiseizure drug chemically classified as a sulfonamide and unrelated to other antiseizure agents. Pharmacological Classes Decreased Central Nervous System Disorganized Electrical Activity - PE Physiologic Effect Anti-epileptic Agent - EPC Established Pharmacologic Class Sulfonamides - CS Carbonic Anhydrase Inhibitors - MoA Mechanism of Action. DETAILED DESCRIPTION OF THE INVENTION Zonisamide being an important and active anti-epileptic agent possessing anti- convulsant and anti-neurotoxic effects a.
Mechanism of action similar to phenytoin. Essentially 100 of the zonisamide dose is absorbed. Active substance zonisamide Pharmaceutical form Capsule hard Strength 25 mg.
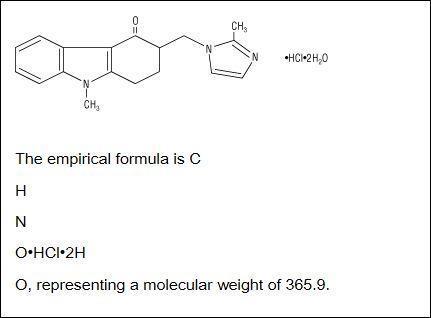
The empirical formula is C8H8N2O3S with a molecular weight of 21223. Parent drug and SMAP can additionally be glucuronidated. Form Capsule hard MA Holder in the RMS neuraxpharm Arzneimittel GmbH Elisabeth-Selbert-Str.
Zonisamide Ad 810 Carbonic Anhydrase Inhibitor Medchemexpress
Analytica Free Full Text Ion Channel Antiepileptic Drugs An Analytical Perspective On The Therapeutic Drug Monitoring Tdm Of Ezogabine Lacosamide And Zonisamide Html

Structures Of Topiramate And Zonisamide Originally Developed As Download Scientific Diagram
Zonisamide Non Selective Na Ca2 Channel Blocker Cas 68291 97 4 Ab120369
Zonisamide 100mg Name Patient Medical Supply Pharmaceutical Export

Zonisamide Capsules 1800petmeds
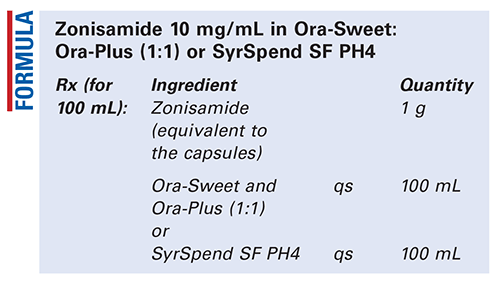
Zonisamide 10 Mg Ml In Ora Sweet Ora Plus 1 1 Or Syrspend Sf Ph4

Structures Of Topiramate And Zonisamide Originally Developed As Download Scientific Diagram
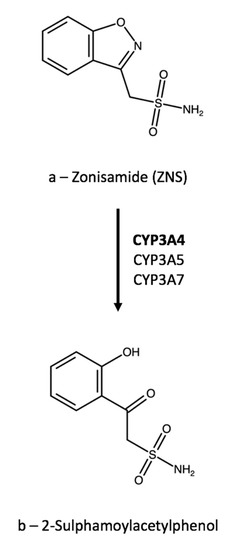
Analytica Free Full Text Ion Channel Antiepileptic Drugs An Analytical Perspective On The Therapeutic Drug Monitoring Tdm Of Ezogabine Lacosamide And Zonisamide Html


No comments:
Post a Comment